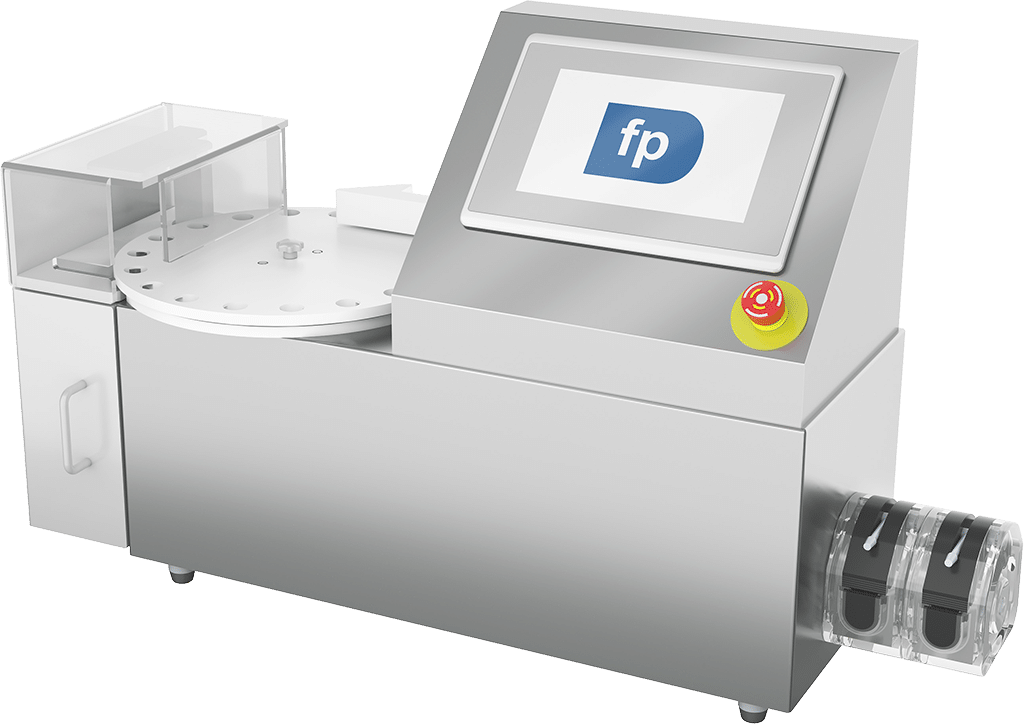
FP Developments provides automation systems purpose-built for 503B outsourcing facilities that demand high throughput, data integrity, and regulatory compliance.
Our modular filling and labeling platforms are designed for cGMP environments and deliver unmatched precision, traceability, and repeatability. Whether you’re expanding production or upgrading existing lines, our solutions are engineered to meet FDA 21 CFR Part 11 and USP <797>/<800> standards with confidence.
Every FP Developments project is a partnership—from system design through validation and service. With more than six decades of experience in automation for pharmaceutical manufacturing, we help your operation run smarter, safer, and faster.