Engineered for efficiency, safety, and compliance, the SterileFill system automates the entire IV syringe repackaging process – from filling and capping to printing, labeling, and bagging. Designed to accommodate a wide range of drugs, syringe types, and fill volumes, SterileFill delivers up to 140 filled 10mL syringes per hour with precision. Built-in quality checks verify fill level, cap placement, and label accuracy on every unit. For enhanced workflow integration, our optional through-the-wall syringe transfer module enables seamless movement from sterile to non-sterile environments without compromising process integrity.
Automated IV Syringe Packaging System
SterileFill & LabelBag Models – Presented by FP Developments
Optional Features
- Throughput: Increased batch capacity behind a modified “2-up” design
- Batch programmability: Run small runs in sequence and understand timing to complete

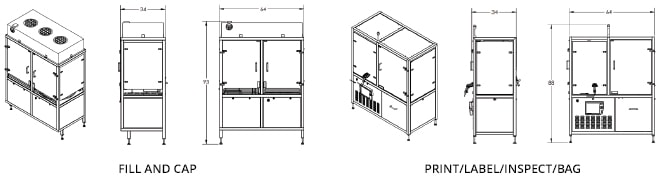
Industry Application & Layout Options
Integrated Fill-Finish Automation with Sterility Assurance
The system automates syringe filling, capping, labeling, and bagging, with built-in verification of fill level, cap presence, and label accuracy. Sterility assurance is supported through validated aseptic processes and optional sterile-to-non-sterile transfer modules.
Modular by Design. Built for USP <797> Compliance
SterileFill’s modular architecture supports deployment across segregated compounding areas, including transitions between buffer rooms (ISO Class 5) and packaging areas, in accordance with USP <797> requirements for airflow, pressure differentials, and contamination control, ensuring environmental integrity in high-risk sterile compounding zones.
IT Specifications
The FP Developments equipment is integrated within the customer’s network infrastructure, which is responsible for overseeing security.
For optimal configuration, the equipment should be placed behind the facility’s firewall. Whenever possible, it is recommended that the equipment be situated in a dedicated local area network (LAN) segment.
To ensure seamless communication between the FP Developments system and the facility’s network, the customer must provide the necessary network infrastructure and details.
Network Requirements & Limitations
- A network speed of 100 Mbps or greater for the LAN or WAN is ideal, with 10 Mbps being the minimum requirement.
- Category 5 or higher cabling should be used from the switch to the jack for all applicable units.
- Own dedicated network port located near the robot’s final placement.
- One static IP address, subnet mask, and gateway address are required.
- DNS or WINS server addresses must be provided for name resolution.
- FP Developments does not support Network Address Translation (NAT) IP configurations. DHCP or DHCP reservations are supported.

Trusted Pharma Packaging Solutions for 60 Years
At FP Developments, we’ve been engineering pharmaceutical packaging equipment for six decades. Customers count on us to deliver on our word, solve complex challenges, and stand behind our work. Every project is a partnership—from design to installation and beyond. Our team is always available to our customers for ongoing service, upgrades, and support throughout the life of your machine.
Customer Testimonials
Contact FP Developments
Ready to enhance your production capabilities with precision and innovation? Whether you’re looking to optimize an existing line, implement cutting-edge automation, or develop a custom solution, FP Developments Inc. is here to help. With over 60 years of expertise in pharmaceutical, biotech, and medical device packaging and machinery, we deliver solutions that meet your unique challenges.
Get in Touch Today
Connect with us to learn more about how we can support your business:
FP Sales:
- Contact: Jeff Denson – Head of Sales & Operations
- Phone: (856) 466-3581
- Email: jdenson@fpdevelopments.com
On-staff Hospital Pharmacist:
- Contact: Jennifer Hillman
- Phone: (210) 887-6628
- Email: jjh_hillman@yahoo.com
Our team is ready to answer your questions, offer insights, and provide tailored solutions to meet your manufacturing and compliance needs.
Let’s create a solution that works for you—today and tomorrow.

