At FP Developments, we’ve been engineering automation and packaging systems for Hospital Pharmacy and 503a environments for over six decades. Our solutions help compounding pharmacies improve efficiency, accuracy, and compliance, while maintaining the highest standards of safety and quality.
Customers count on us to solve complex challenges, deliver on our promises, and stand behind every machine we build. From design to installation to long-term service, every project is a true partnership.
Hospital Pharmacy & 503a
Automation and packaging solutions engineered for compliance, safety, and operational efficiency.
Trusted Hospital Pharmacy & 503a Packaging Solutions for 60 Years
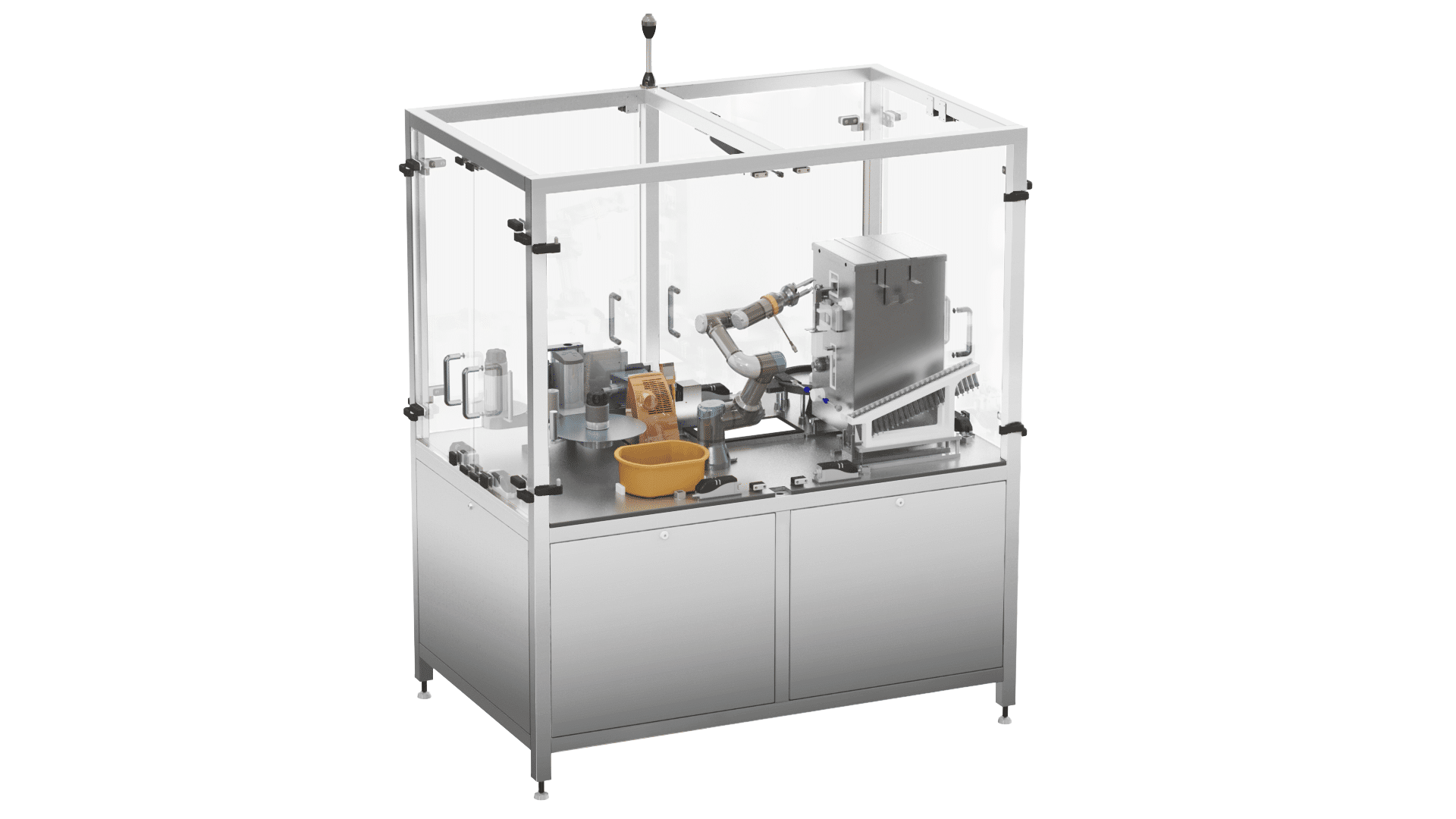
Automated Oral Syringe Filling
Our Jenbot offers oral syringe filling, capping, labeling, and handles a variety of drugs, syringe types, and fill levels.

Automated IV Syringe Filling
Engineered for efficiency, safety, and compliance, our systems automate the entire IV syringe repackaging process.

Desktop Vial Extraction
Tabletop vial extractor: 1,000 vials/hour, <5-min changeovers, safe auto-ejection—fast, accurate, efficient processing.

Automated Syringe Filling Pump
Our Rotary Model pump delivers a streamlined path to USP compliance while strengthening sterility assurance.
Hospital Pharmacy Unit Dose Automation
Our systems are designed to meet your unique processes and requirements, ensuring speed, compliance, and scalability. We offer a range of solutions, including full automation and modular options for filling, stoppering, capping, labeling, verification, inspection, and bagging.
Whether you need compact tabletop systems or high-speed standalone machines, we have a solution to fit your needs and budget. With 60 years of engineering and fabrication experience, we make it easy to find solutions to your toughest challenges while saving you time and money.

Some Frequently Asked Questions
A 503A compounding pharmacy creates patient-specific medications prescribed by a physician, rather than dispensing mass-produced drugs. Unlike retail pharmacies, 503A operations focus on sterile compounding, aseptic technique, and USP <797>/<800> compliance.
USP <797> sets the requirements for sterile compounding environments, while USP <800> addresses the safe handling of hazardous drugs. Hospital pharmacies and compounding labs must meet these to ensure sterility and staff safety.
Certification typically occurs every six months or after major maintenance. Environmental monitoring should be ongoing, tracking viable and nonviable particles to maintain compliance with USP <797>.
Frequent issues include inadequate documentation, poor airflow certification, lack of personnel training, and improper cleaning procedures.
Decisions often depend on staffing, cost, and compliance risk. Outsourcing is increasing as health systems seek consistency, scalability, and validated sterility assurance.
Automation, gravimetric verification, and real-time digital logging are transforming compounding accuracy, reducing waste, and improving traceability.
Couldn’t find your answer? Ask a question
Customer Testimonials
Contact FP Developments
Ready to enhance your production capabilities with precision and innovation? Whether you’re looking to optimize an existing line, implement cutting-edge automation, or develop a custom solution, FP Developments Inc. is here to help. With over 60 years of expertise in pharmaceutical, biotech, and medical device packaging and machinery, we deliver solutions that meet your unique challenges.
Get in Touch Today
Connect with us to learn more about how we can support your business:
FP Sales:
- Contact: Jeff Denson – Head of Sales & Operations
- Phone: (856) 466-3581
- Email: jdenson@fpdevelopments.com
On-staff Hospital Pharmacist:
- Contact: Jennifer Hillman
- Phone: (210) 887-6628
- Email: jjh_hillman@yahoo.com
Our team is ready to answer your questions, offer insights, and provide tailored solutions to meet your manufacturing and compliance needs.
Let’s create a solution that works for you—today and tomorrow.

