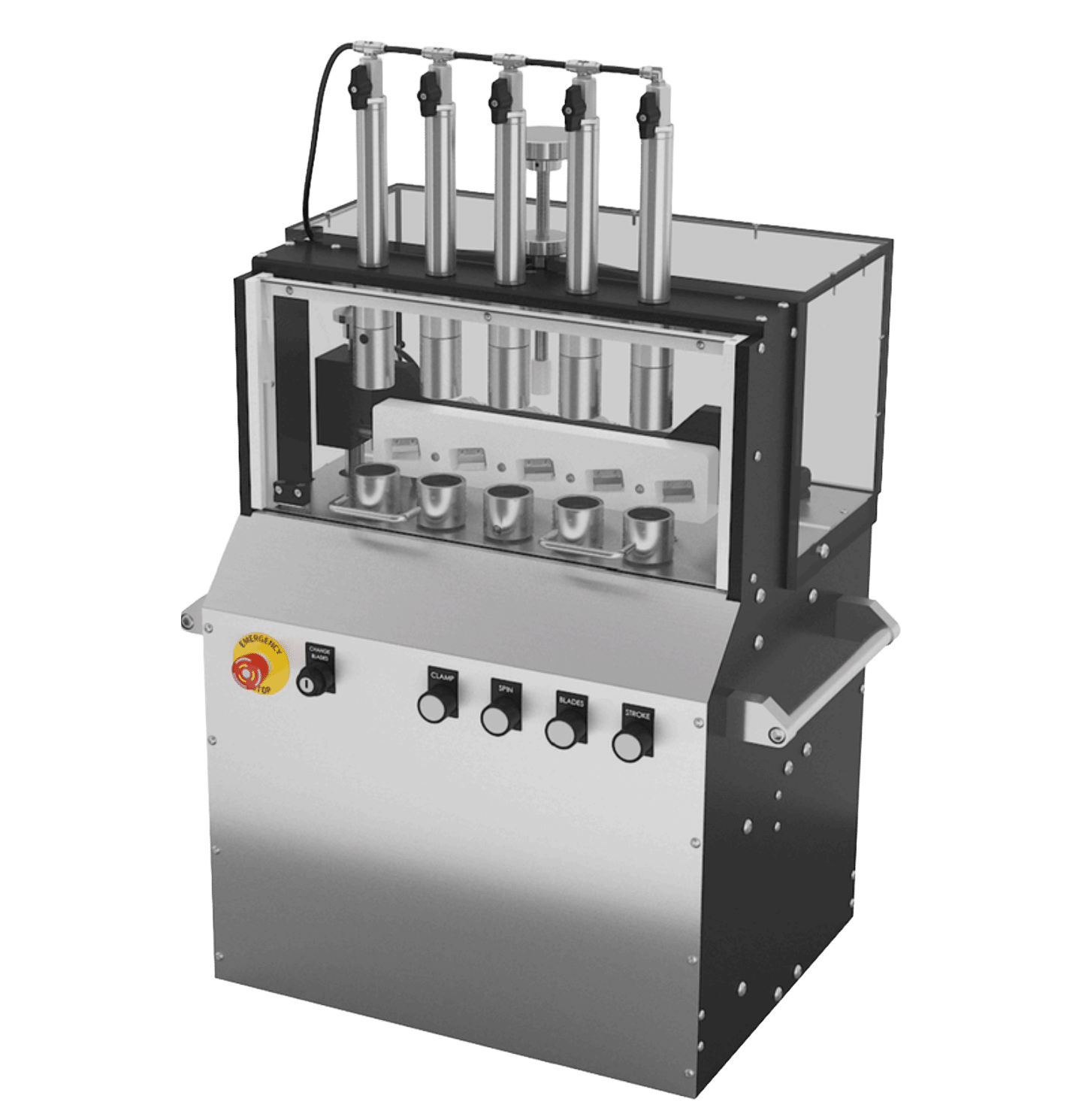
FP Developments partners with pharmaceutical manufacturers and CDMOs to deliver advanced automation systems for filling, labeling, and packaging operations.
Our equipment is designed for scalability, data integrity, and long-term reliability across aseptic and non-aseptic processes. From small-batch R&D to full production lines, we help contract manufacturers achieve consistent quality and compliance with every run.
With over 60 years of engineering experience, we understand the operational challenges of pharma production—and we build systems that improve efficiency, traceability, and throughput without compromising safety.